Li-Na Song, Wei Zhang, Ying Wang, Xin Ge, Lian-Chun Zou, Huan-Feng Wang, Xiao-Xue Wang, Qing-Chao Liu, Fei Li, Ji-Jing Xu*. Nat. Commun. 2020, 11, 2191. DOI: 10.1038/s41467-020-15712-z. Published 4 May 2020
In this work, the authors clearly illustrated that the single atom Co catalyst with the maximum atomic utilization could regulate the electrochemical reaction path of the electrode and greatly improved the reaction kinetics and energy efficiency of the lithium-oxygen (Li–O2) batteries. This work not only provides insights into the design and development of highly efficient catalysts for Li–O2 batteries, but also opens a new allpication for single atom catalysts.

Figure 1. Schematic illustration showing the synthesis of N-HP-Co SACs.
Rechargeable Li–O2 batteries provide a theoretical specific energy density (3500 Wh kg−1) that is 5–10 times higher than that of conventional Li-ion batteries, thus making the it the destination of choice for vehicle applications with an attraction of disruptive and revolutionary battery technology. However, due to the limitation of the sluggish kinetics of ORR and OER, the low practical capacity, inferior rate performance, low energy efficiency and poor cycle life of Li–O2 batteries are still far from their industrial application. A variety of highly-efficient, stable cathode catalysts have been developed to solve the problem of sluggish kinetics of ORR and OER by reducing the battery polarization, however, the low catalytic efficiency are still a difficult problem to overcome. Furthermore, it is not enough to improve the catalytic activity of the catalyst in the lithium oxygen battery. Regulating the intrinsic electrochemical reaction process of the battery by catalyst would provide the fundamental strategy to improve the ORR and OER kinetics, unfortunately, less attention has been paid to this at present. Single atom catalysts have attracted much attention due to their excellent catalytic activity in many fields originated from the particular electronic structure and maximum atomic utilization of such materials. It is very meaningful but challenging to improve the kinetics of electrochemical reaction and further study the regulating mechanism of catalyst on electrochemical process by introducing the single atom catalyst into Li–O2 batteries.
The group of Prof. Ji-Jing Xu constructed a hollow N-doped carbon sphere with isolated single Co sites (N-HP-Co SACs) as cathode catalyst for Li–O2 batteries (Figure 1). Benefiting from the combining merits of the maximum exposed single atom active sites of CoN 4 and the homogeneous distribution of atomic sites on carbon sphere, the ORR and OER kinetics of Li–O2 batteries with these N-HP-Co SACs were greatly enhanced, and thus the improved energy efficiency. Besides, authors explicitly pointed out that atomically dispersed active sites were capable of inducing the homogeneous nucleation and epitaxial growth of the discharge products to form micrometer-sized flower-like lithium peroxide. During the charging process, the weaken adsorption energy of CoN4 towards the intermediate LiO2 would induce the reaction to convert from a two-electron reaction to a single electron reaction (Figure 2). Owing to the favorable regulation of electrochemical reaction by single atom catalysts, the battery operated with high round-trip efficiency (86.2%) and long-term stability (218 days), even superior to the commercial platinum/carbon noble metal catalyst.
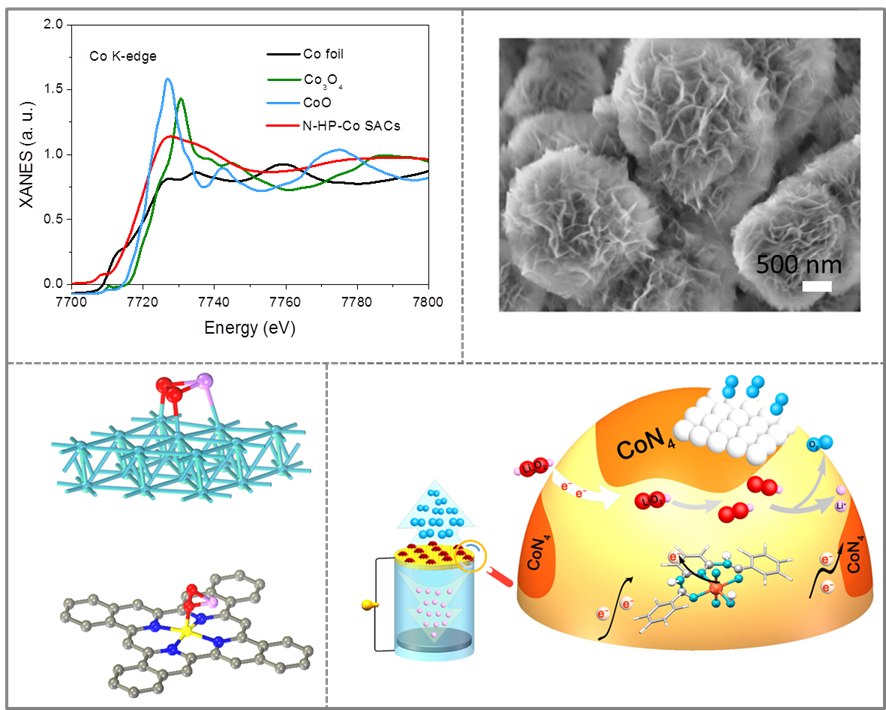
Figure 2. Schematic illustration showing the discharge-charge mechanisms of the N-HP-Co SAC-catalysed Li-O2 batteries.
This paper reports a remarkable cooperative effort among 7 laboratories in two countries. Prof. Ji-Jing Xu from Jilin University is the corresponding author of the paper. Li-Na Song from Jilin University, Prof. Wei Zhang from Jilin University and Basque Foundation for Science, associate Prof. Ying Wang from Changchun Institute of Applied Chemistry Chinese Academy of Sciences are the co-first authors of this work. Other co-authors include Dr. Xin Ge, Dr. Lian-Chun Zou, Dr. Huan-Feng Wang, Dr. Xiao-Xue Wang, Dr. Fei Li from Jilin University, and Dr. Qing-Chao Liu from Zhengzhou University.
Read more at Nat. Commun.:
https://doi.org/10.1038/s41467-020-15712-z
The paper was highlighted by Wechat media platform:
https://mp.weixin.qq.com/s/NJj2ko2qFAdnVTukLzoxNA