Malin Lia,b, Xiaoxue Wanga, Fei Lia, Lijun Zhenga, Jijing Xua,b* and Jihong Yua,b*. Adv. Mater. DOI: 10.1002/adma.201907098.
a. State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
b. International Center of Future Science, Jilin University, Changchun 130012, P. R. China
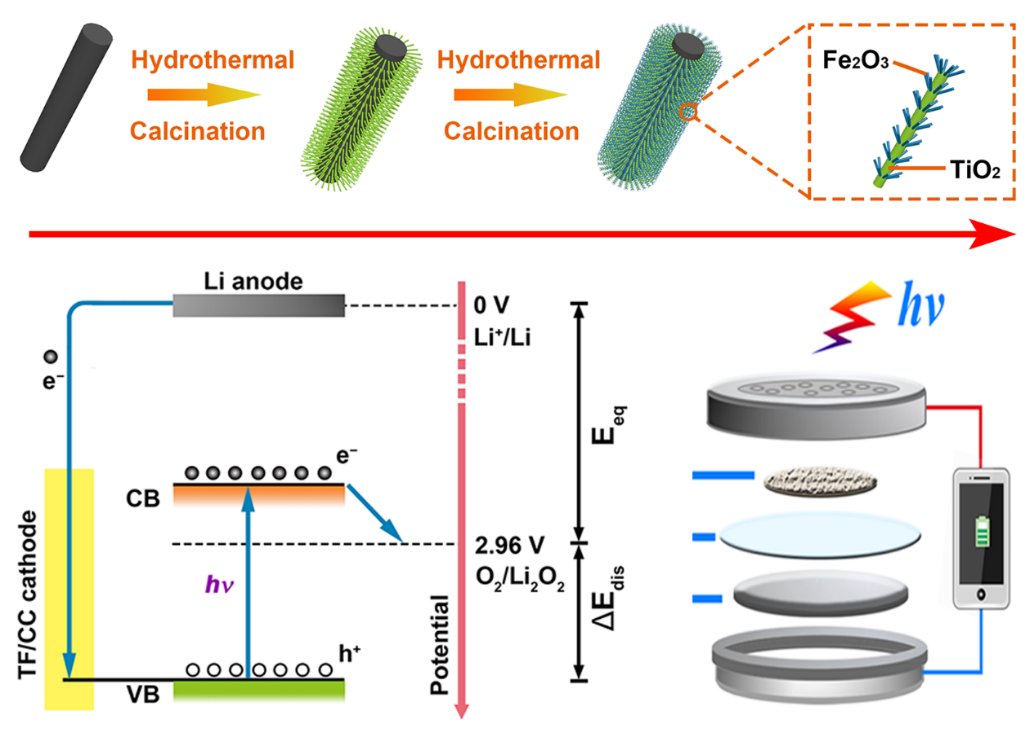
Photo-assisted charging is considered an effective approach to reducing the overpotential in lithium-oxygen (Li-O2) batteries. However, the utilization of photoenergy during the discharge process in a Li-O2 system has been rarely reported, and the functional mechanism of such a process remains unclear. Herein, a novel bifunctional photo-assisted Li-O2 system has been established by employing a hierarchical TiO2-Fe2O3 heterojunction, in which the photo-generated electrons and holes played key roles in reducing the overpotential in the discharging and charging processes, respectively. Moreover, the morphology of the discharge product (Li2O2) could be modified via the dense surface electrons of the cathode under illumination, resulting in the promoted decomposition kinetics of Li2O2 during the charging progress. Accordingly, the output and input energies of the battery could be tuned by illumination, giving an ultralow overpotential of 0.19 V between the charge and discharge plateaus with excellent cyclic stability (retaining a round-trip efficiency of ~86% after 100 cycles). The investigation of the bifunctional photo-assisted process presented in this work provides a significant insight into the mechanism of the photo-assisted Li-O2 battery and addresses the overpotential bottleneck in this system.