Feng Penga,b,#, Xianqi Songc,d,#, Chang Liud,e,f, Quan Lic,d,e,f,*, Maosheng Miaob, Changfeng Cheng,* and Yanming Mac,d,e,*. Nat. Commun., 11, 5227, 2020. DOI: 10.1038/s41467-020-19107-y
a. College of Physics and Electronic Information & Henan Key Laboratory of Electromagnetic Transformation and Detection, Luoyang Normal University, 471022 Luoyang, China.
b. Department of Chemistry and Biochemistry, California State University Northridge, Northridge, CA 91330-8262, USA.
c. State Key Laboratory of Superhard Materials, College of Physics, Jilin University, 130012 Changchun, China.
d. Innovation Center for Computational Methods & Software, College of Physics, Jilin University, 130012 Changchun, China.
e. International Center of Future Science, Jilin University, 130012 Changchun, China.
f. Key Laboratory of Automobile Materials of MOE and Department of Materials Science, College of Materials Science and Engineering, Jilin University, 130012 Changchun, China.
g. Department of Physics and Astronomy, University of Nevada, Las Vegas, NV 89154, USA.
# These authors contributed equally.
An enduring geological mystery concerns the missing xenon problem, referring to the abnormally low concentration of xenon compared to other noble gases in Earth’s atmosphere. Identifying mantle minerals that can capture and stabilize xenon has been a great challenge in materials physics and xenon chemistry. Here, using an advanced crystal structure search algorithm in conjunction with first-principles calculations we find reactions of xenon with recently discovered iron peroxide FeO2, forming robust xenon-iron oxides Xe2FeO2 and XeFe3O6 with significant Xe-O bonding in a wide range of pressure-temperature conditions corresponding to vast regions in Earth’s lower mantle. Calculated mass density and sound velocities validate Xe-Fe oxides as viable lower-mantle constituents. Meanwhile, Fe oxides do not react with Kr, Ar and Ne. It means that if Xe exists in the lower mantle at the same pressures as FeO2, xenon-iron oxides are predicted as potential Xe hosts in Earth’s lower mantle and could provide the repository for the atmosphere’s missing Xe. These findings establish robust materials basis, formation mechanism, and geological viability of these Xe-Fe oxides, which advance fundamental knowledge for understanding xenon chemistry and physics mechanisms for the possible deep-Earth Xe reservoir.
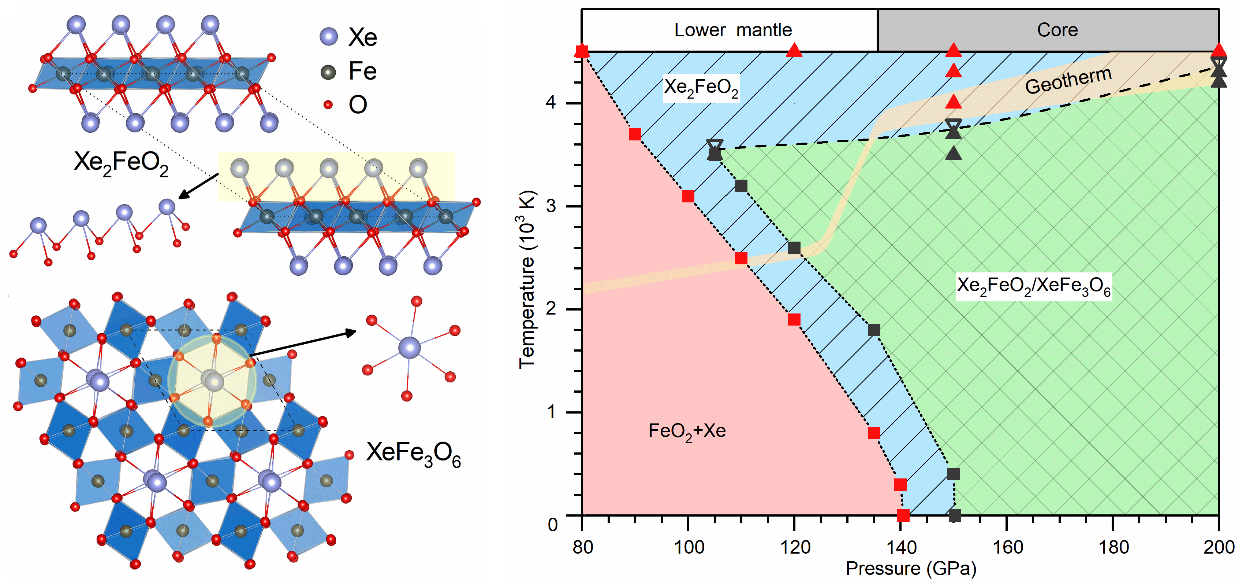
Left panel: Structures of Xe-Fe oxides; Right panel: The Pressure-temperature (P-T) phase diagram of the Xe-FeO2 system.
This paper reports a remarkable cooperative effort among 7 laboratories in two countries. Prof. Quan Li and Prof. Yanming Ma, from Jilin University and Prof. Changfeng Chen, from University of Nevada, Las Vegas are the corresponding authors of the paper. Prof. Feng Peng and Dr. Xianqi Song are the first authors from Luoyang Normal University and Jilin University, respectively. Other co-author includes Dr. Chang Liu from Jilin University and Prof. Maosheng Mao from California State University Northridge.
Read more at https://www.nature.com/articles/s41467-020-19107-y