Guan-Yu Qiao, Dehui Guan, Shuai Yuan, Heng Rao, Xiao Chen, Jia-Ao Wang, Jun-Sheng Qin,* Ji-Jing Xu,* and Jihong Yu*
Guan-Yu Qiao State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
Dehui Guan State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
Shuai Yuan State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210023, P.R. China
Heng Rao State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
Jia-Ao Wang Department of Chemistry and the Oden Institute for Computational Engineering and Sciences, The University of Texas at Austin, Austin, Texas 78712-0165, United States
Jun-Sheng Qin* State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
International Center of Future Science, Jilin University, Changchun 130012, P.R. China
*qin@jlu.edu.cn
Ji-Jing Xu* State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
International Center of Future Science, Jilin University, Changchun 130012, P.R. China
*jijingxu@jlu.edu.cn
Jihong Yu* State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
International Center of Future Science, Jilin University, Changchun 130012, P.R. China
*jihong@jlu.edu.cn
Metal halide perovskite quantum dots, with high light-absorption coefficients and tunable electronic properties, have been widely studied as optoelectronic materials, but their applications in photocatalysis are hindered by their insufficient stability because of the oxidation and agglomeration under light, heat, and atmospheric conditions. To address this challenge, herein, we encapsulated CsPbBr3 nanocrystals into a stable iron-based metal–organic framework (MOF) with mesoporous cages (∼5.5 and 4.2 nm) via a sequential deposition route to obtain a perovskite-MOF composite material, CsPbBr3@PCN-333(Fe), in which CsPbBr3 nanocrystals were stabilized from aggregation or leaching by the confinement effect of MOF cages. The monodispersed CsPbBr3 nanocrystals (4–5 nm) within the MOF lattice were directly observed by transmission electron microscopy and corresponding mapping analysis and further confirmed by powder X-ray diffraction, infrared spectroscopy, and N2 adsorption characterizations. Density functional theory calculations further suggested a significant interfacial charge transfer from CsPbBr3 quantum dots to PCN-333(Fe), which is ideal for photocatalysis. The CsPbBr3@PCN-333(Fe) composite exhibited excellent and stable oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) catalytic activities in aprotic systems. Furthermore, CsPbBr3@PCN-333(Fe) composite worked as the synergistic photocathode in the photoassisted Li–O2 battery, where CsPbBr3 and PCN-333(Fe) acted as optical antennas and ORR/OER catalytic sites, respectively. The CsPbBr3@PCN-333(Fe) photocathode showed lower overpotential and better cycling stability compared to CsPbBr3 nanocrystals or PCN-333(Fe), highlighting the synergy between CsPbBr3 and PCN-333(Fe) in the composite.
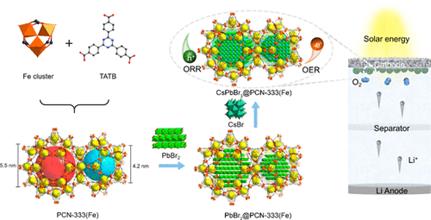
Figure 1. Schematic View of the Preparation of PCN-333(Fe) and CsPbBr3@PCN-333(Fe) Compositea
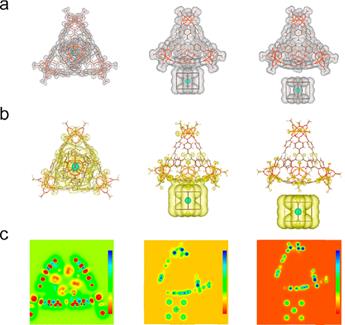
Figure 2. (a) Charge density of CsPbBr3 QDs in the cage, outside the cage, and far away. (b) Charge density difference of CsPbBr3 QDs in the cage, outside the cage, and far away. (c) Selected interested planar 2D DCD of CsPbBr3 QDs in the cage, outside the cage, and far away.
Related links: https://doi.org/10.1021/jacs.1c05907