Advanced Materials https://doi.org/10.1002/adma.202300053
Hang Yang, Tengsheng Zhang, Duo Chen, Yicheng Tan, Wanhai Zhou, Li Li, Wei Li, Guangshe Li, Wei Han*, Hong Jin Fan*, Dongliang Chao*
In the literature, Zn–Mn aqueous batteries (ZMABs) confront abnormal capacity behavior, such as capacity fluctuation and diverse “unprecedented performances.” Because of the electrolyte additive-induced complexes, various charge/discharge behaviors associated with different mechanisms are being reported. However, the current performance assessment remains unregulated, and only the electrode or the electrolyte is considered. The lack of a comprehensive and impartial performance evaluation protocol for ZMABs hinders forward research and commercialization. Here, a pH clue (proton-coupled reaction) to understand different mechanisms is proposed and the capacity contribution is normalized. Then, a series of performance metrics, including rated capacity (Cr) and electrolyte contribution ratio from Mn2+ (CfM), are systematically discussed based on diverse energy storage mechanisms. The relationship between Mn (II) ↔ Mn (III) ↔ Mn (IV) conversion chemistry and protons consumption/production is well-established. Finally, the concrete design concepts of a tunable H+/Zn2+/Mn2+ storage system for customized application scenarios, opening the door for the next-generation high-safety and reliable energy storage system, are proposed.

Figure 1. Summary of charge/discharge mechanisms and abnormal behaviors for ZMABs. a) Main progress for ZMABs. Light gray, dark red, gray, and
dark blue arrows represent the prototype of the primary Zn–MnO2 battery, iteration of types of Mn2+ ion additives, side reactions of Mn2+ ion, and new
battery systems associated with Mn2+ ions, respectively. b) Schematics of abnormal capacity behavior and possible reaction mechanisms for ZMABs.
The red part of the curve corresponds to the rapid capacity increase from the electrolyte. Gray represents reversible ion (de)intercalation. Dark blue
indicates capacity decay due to irreversible phase transitions.
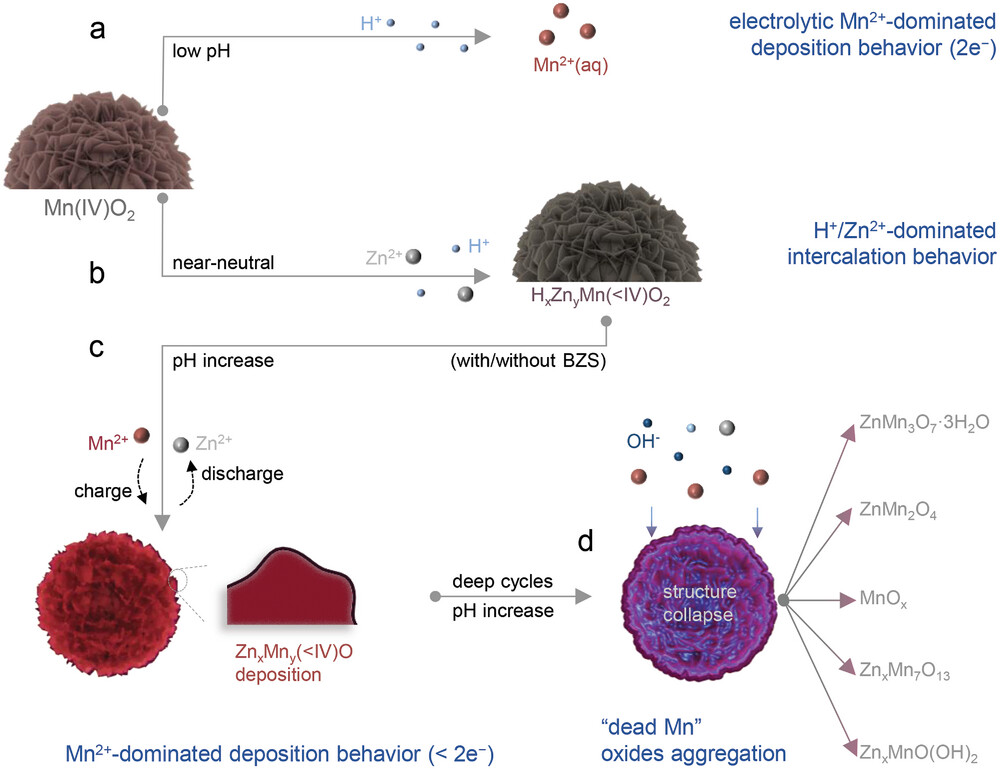
Figure 2. Illustration of the H+/Zn2+/Mn2+-dominated electrochemical process at difffferent pH environments. a) Schematic diagram of electrolytic
Mn2+-dominated electrochemical behavior at low pH. b) Schematic diagram of H+/Zn2+-dominated electrochemical behavior at near-neutral pH.
c) Schematic diagram of Mn2+-dominated deposition at near-neutral pH. d) Schematic diagram of “dead Mn” oxides aggregation and the morpho�
logical deterioration at near-neutral pH.
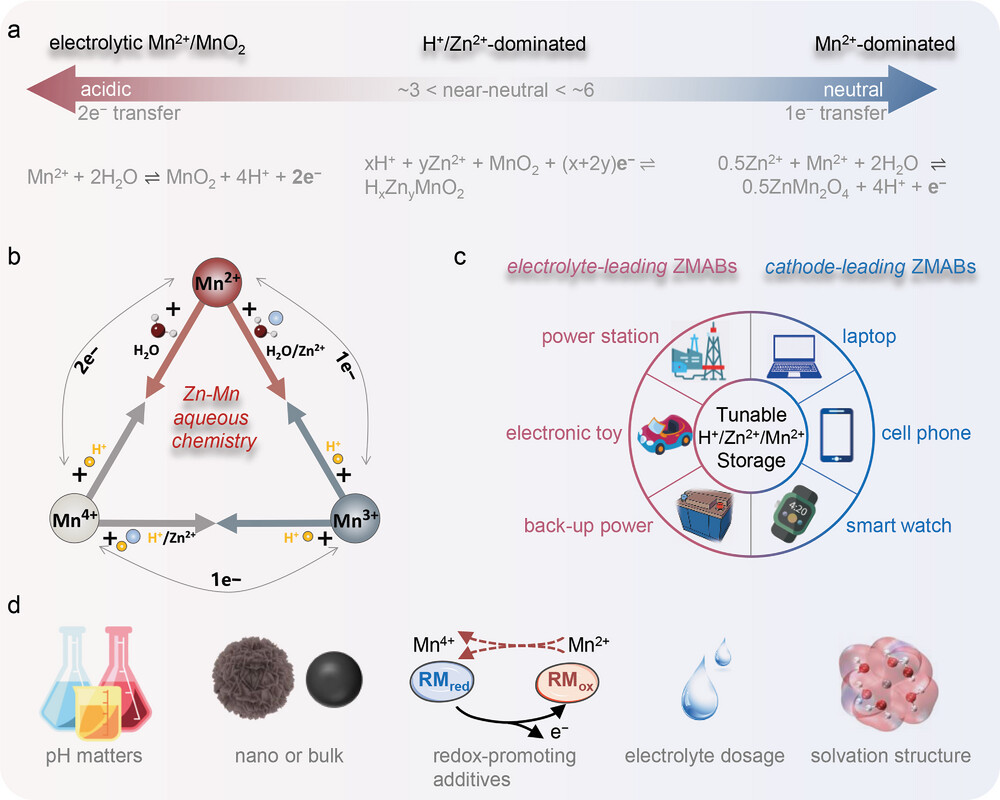
Figure 3. Overall conclusion and perspective to future commercialization. a) Summary of pH-dependent ZMABs. b) Zn–Mn aqueous chemistry with
the relationship between (II) ↔ Mn (III) ↔ Mn (IV) conversion and protons consumption/production. c) The potential device application scenarios of
ZMABs. d) Proposed future research directions in regulating ZMABs.